Structure of the research program
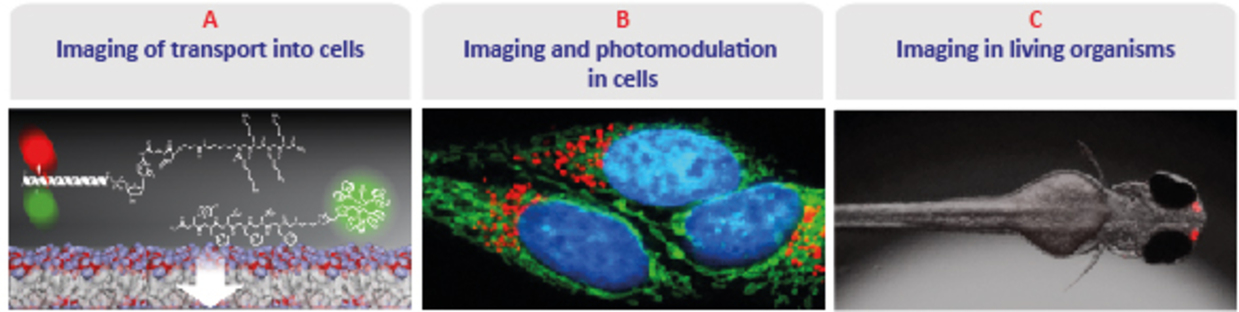
Project area A: Imaging of transport into cells
A1 Multifunctional transport moieties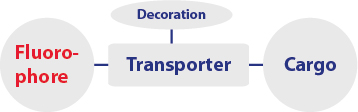
Project A1 involves the synthesis and characterization of multifunctional transport moieties adjusted for the cellular uptake in an organelle- and organ-specific manner.
A3 Wavelength-shifting siRNA probes
In project A3, wavelength-shifting RNA probes for imaging RNA delivery, transport and processing in living cells and animals without interfering significantly the silencing efficiacy are being developed.
A4 Far-red and near infrared (NIR) emitters for imaging in living cells
Project A4 focuses on zinc(II) dipyrrinato metal complexes, as far-red and near infrared emitting dyes that are being applied in live cell imaging experiments. The chromophoric unit can further functionalized in order to sense biological molecules.
Project area B: Imaging and photomodulation in cells
B4 Photocontrol and imaging of membrane-active peptides
The challenge of project B4 is to generate cell penetrating peptides that feature both, an efficient photocontrol via the introduction of a diarylethene photoswitch and a high fluorescence, allowing to use them as treatment and diagnostic tools. In addition, an electron transporting transmembrane peptide is being developed that allows an selective electron flow across the cell membrane triggered by photoinduction.
B5 Supramolecular DNA architectures for studying early signaling events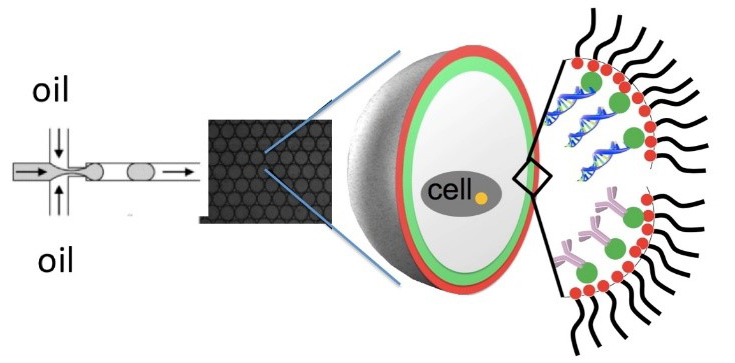
Project B5 involves the development of surface-immobilized DNA origami nanostructure (MOSAIC) for elucidation of early cell signaling events occuring during EGFR signaling and mast cell activation. Furthermore, fluorescent silica nanoparticles are being applied to create novel cell-instructive 3D micro-bioreactors bearing EGF ligands to investigate the fate of encapsulated eukaryotic cells.
Project are C: Imaging in living organisms
C1 Glucose and small molecule sensing
Project C1 focuses on novel injectable sensors for small molecule detection, which are based on supramolecular nanoparticle-constructs bearing genetically-engineered metabolite-binding proteins. With this molecular toolbox, metabolism during embryonic development, as well as metabolic alterations in the zebrafish model can be studied with the idea to model human diseases.
C2 In vivo imaging using multimodal carrier systems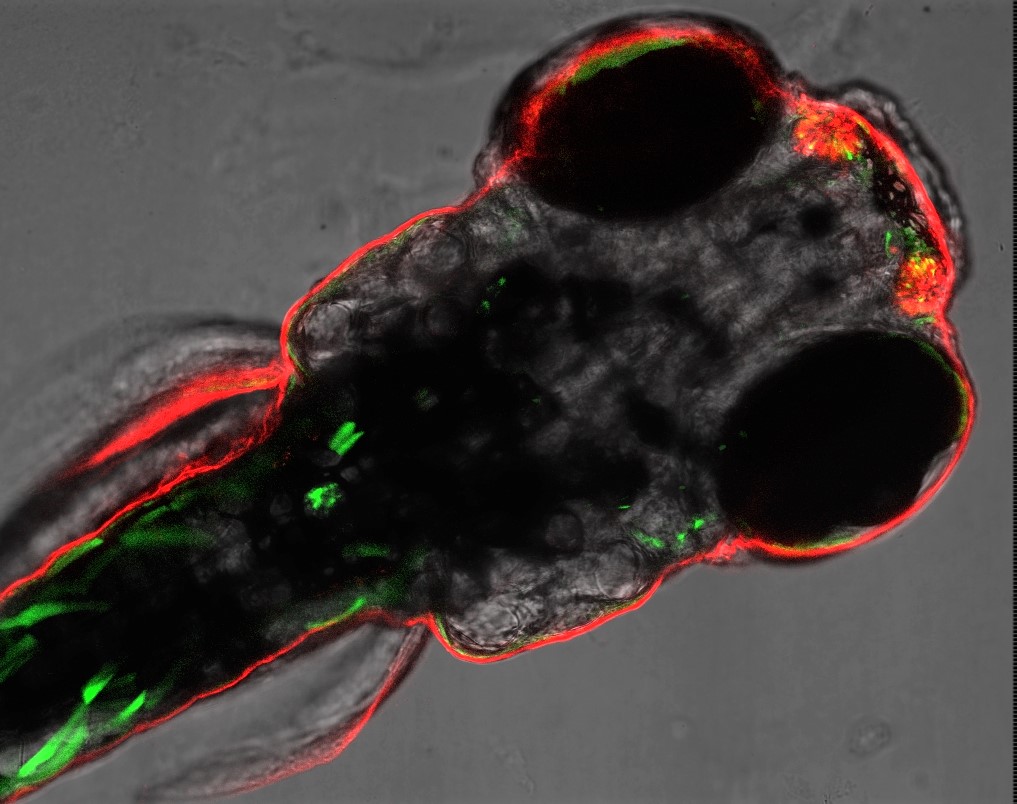
The challenge of project C2 lies in the development of multimodall carrier systems applicable on hybrid in vivo imaging platforms. Systematic screenings for tissue specificity in zebrafish and mice are required to identify interesting candidates.
C3 cnRNA imaging in the CNS
The overall goal of project C3 is to develop efficient fluorencence based methods to detect the presence of short and/or low abundant ncRNAs in fixed cells and in zebrafish brains.
