MSc. Andrii Hrebonkin
- B4
- Group: Prof. Ulrich
- Room: CS / B30.42 / R501
- Phone: 47223
- andrii hrebonkin ∂does-not-exist.kit edu
My project focuses on the development of novel photopharmacology agents as anticancer drug candidates. These agents shall be modified with chemical moieties allowing the fluorescence readout to help in structure-activity relationship (SAR) studies and in the mechanism-of-action (MoA) investigations. This feature will support pharmacokinetics (PK) characterization of my substances and make easier photopharmacological therapy optimization on the cellular, tissue, and whole-organism levels. I continue exploring the application of diarylethene-based photoswitches (DAE) in the backbones of the cyclic bioactive peptides (Figure 1). This approach has been pioneered at KIT by Dr. O. Babii, who developed a range of antimicrobial and oncolytic photopharmacology agents based on a decameric cyclic β-hairpin scaffold of antimicrobial peptide Gramicidin S and of Dr. T. Schober, who recently used DAE in fluorescently-labeled photoswitchable cyclic arginine-rich cell-penetrating peptides.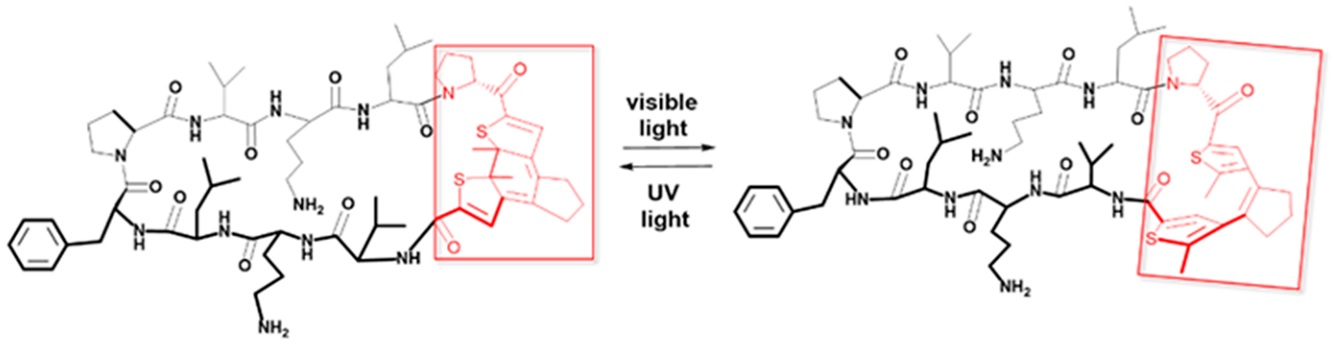
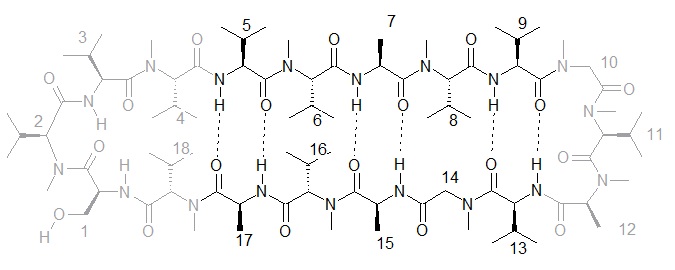
In my Ph.D. project, I combine both approaches (DAE in a cyclic bioactive peptide, and fluorescence labeling) and select as the main target a different to GS parent peptide, Gymnopeptide A (Figure 2), which had been reported to possess anticancer activities in the nanomolar range.